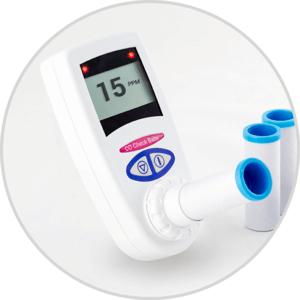
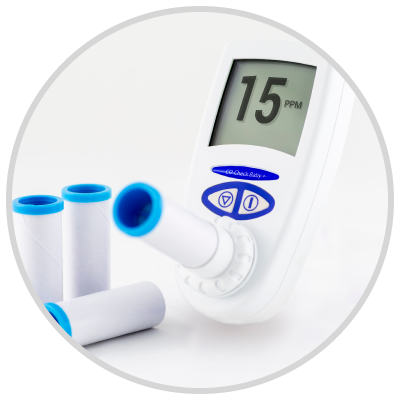
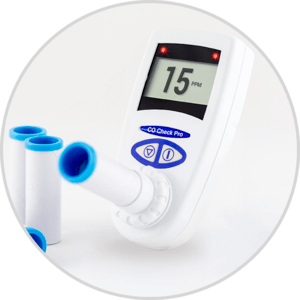
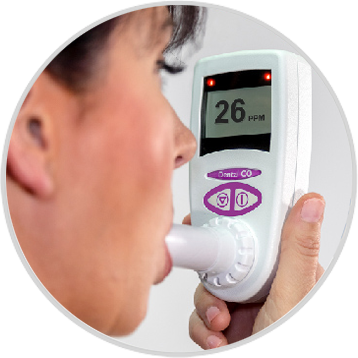
We are pleased to announce that the CO Check Pro carbon monoxide monitor for CO breath testing has gained FDA 510K approval for sale in the USA for use in smoking cessation.
Cigarette smoking is the leading cause of preventable disease and death in the United States, accounting for more than 480,000 deaths each year (approximately 1 in 5 deaths) of which more than 140,000 of these deaths are from second hand smoke(1).
The United States has a strong focus on smoking cessation encouraging current smokers to quit and highlighting the associated health benefits. Carbon monoxide breath testing is a great and invaluable tool in assisting smokers to quit. The CO Check Pro with its clear display of a smokers exhaled carbon monoxide levels in parts per million and % carboxyhemoglobin levels along with visual coloured lights on the levels of breath CO recorded act as great motivational tool in smoking cessation.
The ease of use of the CO Check Pro, ensures tests can be performed quickly and efficiently assisting busy healthcare professionals in their role as smoking cessation advisers. FDA approval will allow health care professionals throughout the United States to use the CO Check Pro to assist with smoking cessation and reduce the burden of preventable deaths and disease associated with smoking.
Approval of the CO Check Pro for the U.S market further increases MD Diagnostics footprint in the global smoking cessation market.
(1)US Department of Health and Human Services. The Health Consequences of Smoking – 50 years of progress a Report of the Surgeon General.
![]()